





Our Flagship Product

Each Uncoated dispersible Tablet Contains: Cefexime Trihydrate IP equ. To Cefexime 200MG
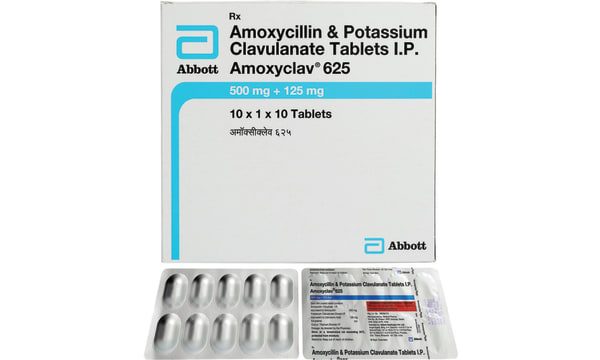
Each film coated contains: Amoxycilllin Trihydrate IPeq. to Amoxycillin 500mg,Potassium Clavulanate IP (as Potassium Clavulanate Diluted IP) eq. to Clavulanic Acid 125mg. Colour - Titanium Dioxide IP
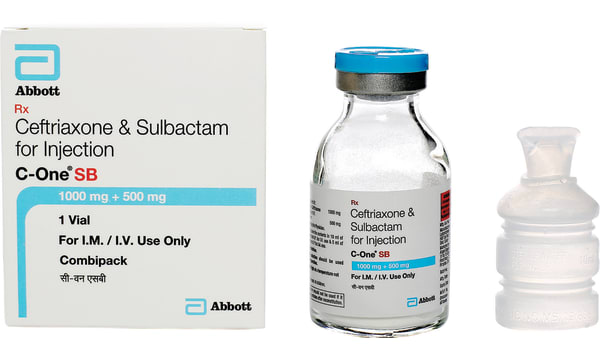
Each vial contains:Ceftriaxone Sodium IP 1.5gms sterileeq. to ceftriaxone 1000mg Sulbactam Sodium USP sterile eq. to Sulbactam 500mg

Each vial contains : Sterile Ceftriaxone Sodium U.S.P. equivalent to Anhydrous Ceftriaxone 1000 mg

Each film coated tablet contains: Ciprofloxacin Hydrochloride IP equivalent to Ciprofloxacin 250mg Colour Titanium Dioxide IP

Each film coated tablet contains Ciprofloxacin Hydrochloride IP equivalent to Ciprofloxacin 500mg
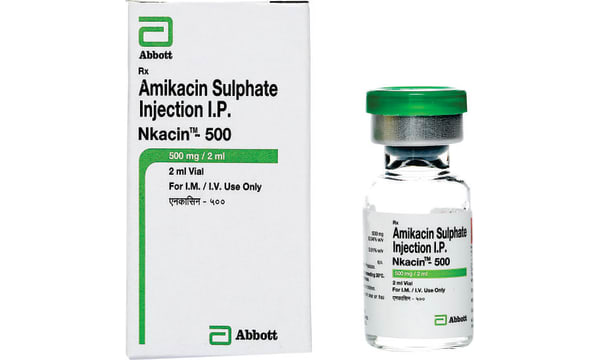
Each 2ml contains Amikacin Sulphate I.P. Equivalent to Amikacin500mg, Methyl Paraben I.P.0.08% w/v Propyl Paraben(As preservative) I.P.0.02% w/v Water for injection I.P. q.s.

Each 5 ml contains: Dextromethorphan Hydrobromide IP
10mg, Chlorpheniramine Maleate IP 2mg, In a flavoured
base q.s. .
Quantity: 100ml
Mrp: 95.71

Composition: Rabeprazole Sodium IP 20mg (As Enteric Coated Pellets), Domperidone IP 30mg (As Sustained Release Pellets)
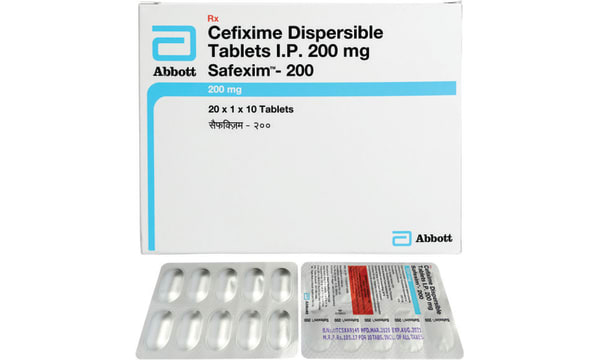
Each Dispersible tablet contains: Cefixime (as Trihydrate) 10Tabs 500 105.17 USP eqv.to Anhydrous Cefixime 200mg Excipients q.s.

Each film coated tablet contains Azthromycin Dihydrate IP eqv. to Azithromycin -500mg. Colour -Yellow Oxide of Iron & Titanium Dioxide IP

Each vials contains: Sterile Ceftrioxone Sodium IP Equivalent to Ceftrioxone 250mg Sterile Sulbactum Sodium USP equivalent to Sulbactum 125mg.